How are water molecules and ice molecules different? The structure of water This complex molecule
Custom search
Water structure
Ph.D. O.V. Mosin
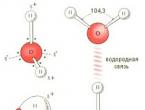
A water molecule is a small dipole containing positive and negative charges at its poles. Since the mass and charge of the oxygen nucleus is greater than that of the hydrogen nuclei, the electron cloud is pulled towards the oxygen nucleus. In this case, the hydrogen nuclei are exposed. Thus, the electron cloud has a non-uniform density. There is a lack of electron density near the hydrogen nuclei, and on the opposite side of the molecule, near the oxygen nucleus, there is an excess of electron density. It is this structure that determines the polarity of the water molecule. If you connect the epicenters of positive and negative charges with straight lines, you get a three-dimensional geometric figure - a regular tetrahedron.
 The structure of a water molecule (picture on the right)
The structure of a water molecule (picture on the right)
Due to the presence of hydrogen bonds, each water molecule forms a hydrogen bond with 4 neighboring molecules, forming an openwork mesh frame in the ice molecule. However, in the liquid state, water is a disordered liquid; These hydrogen bonds are spontaneous, short-lived, quickly break and form again. All this leads to heterogeneity in the structure of water.
 Hydrogen bonds between water molecules (picture below left)
Hydrogen bonds between water molecules (picture below left)
The fact that water is heterogeneous in composition was established long ago. It has long been known that ice floats on the surface of water, that is, the density of crystalline ice is less than the density of liquid.
For almost all other substances, the crystal is denser than the liquid phase. In addition, even after melting, with increasing temperature, the density of water continues to increase and reaches a maximum at 4C. Less well known is the anomaly of water compressibility: when heated from the melting point up to 40C, it decreases and then increases. The heat capacity of water also depends nonmonotonically on temperature.
In addition, at temperatures below 30C, with an increase in pressure from atmospheric to 0.2 GPa, the viscosity of water decreases, and the self-diffusion coefficient, a parameter that determines the speed of movement of water molecules relative to each other, increases.
For other liquids, the relationship is the opposite, and almost nowhere does it happen that some important parameter behaves non-monotonically, i.e. first increased, and after passing a critical value of temperature or pressure decreased. An assumption arose that in fact water is not a single liquid, but a mixture of two components that differ in properties, for example, density and viscosity, and therefore structure. Such ideas began to arise at the end of the 19th century, when a lot of data on water anomalies accumulated.
Whiting was the first to propose the idea that water consists of two components in 1884. His authorship is cited by E.F. Fritsman in the monograph “The Nature of Water. Heavy Water,” published in 1935. In 1891, V. Rengten introduced the concept of two states of water, which differ in density. After it, many works appeared in which water was considered as a mixture of associates of different compositions (hydrols).
When the structure of ice was determined in the 1920s, it turned out that water molecules in the crystalline state form a three-dimensional continuous network in which each molecule has four nearest neighbors located at the vertices of a regular tetrahedron. In 1933, J. Bernal and P. Fowler suggested that a similar network exists in liquid water. Since water is denser than ice, they believed that the molecules in it are arranged not like in ice, that is, like silicon atoms in the mineral tridymite, but like silicon atoms in a denser modification of silica, quartz. The increase in the density of water when heated from 0 to 4C was explained by the presence of the tridymite component at low temperatures. Thus, Bernal Fowler's model retained the element of two-structure, but their main achievement was the idea of a continuous tetrahedral network. Then the famous aphorism of I. Langmuir appeared: “The ocean is one big molecule.” Excessive specification of the model did not increase the number of supporters of the unified grid theory.
It was not until 1951 that J. Pople created a continuous grid model, which was not as specific as Bernal Fowler's model. Pople imagined water as a random tetrahedral network, the bonds between the molecules in which are curved and have different lengths. Pople's model explains the compaction of water during melting by the bending of bonds. When the first definitions of the structure of ices II and IX appeared in the 60-70s, it became clear how the bending of bonds can lead to compaction of the structure. Pople's model could not explain the non-monotonic dependence of water properties on temperature and pressure as well as two-state models. Therefore, the idea of two states was shared by many scientists for a long time.
But in the second half of the 20th century it was impossible to fantasize about the composition and structure of hydrols as they did at the beginning of the century. It was already known how ice and crystalline hydrates work, and they knew a lot about hydrogen bonding. In addition to continuum models (Pople's model), two groups of mixed models have emerged: cluster and clathrate. In the first group, water appeared in the form of clusters of molecules connected by hydrogen bonds, which floated in a sea of molecules not involved in such bonds. The second group of models treated water as a continuous network (usually called a framework in this context) of hydrogen bonds that contained voids; they contain molecules that do not form bonds with the molecules of the framework. It was not difficult to select the properties and concentrations of two microphases of cluster models or the properties of the framework and the degree of filling of its voids of clathrate models in order to explain all the properties of water, including the famous anomalies.
Among the cluster models, the most striking was the model of G. Nemeti and H. Sheragi: The pictures they proposed, depicting clusters of bound molecules floating in a sea of unbound molecules, were included in many monographs.
The first model of the clathrate type was proposed in 1946 by O.Ya. Samoilov: in water, a network of hydrogen bonds similar to hexagonal ice is preserved, the cavities of which are partially filled with monomer molecules. L. Pauling in 1959 created another option, suggesting that the basis of the structure could be a network of bonds inherent in some crystalline hydrates.
During the second half of the 60s and the beginning of the 70s, a convergence of all these views was observed. Variants of cluster models appeared in which molecules in both microphases are connected by hydrogen bonds. Proponents of clathrate models began to admit the formation of hydrogen bonds between void and framework molecules. That is, in fact, the authors of these models consider water as a continuous network of hydrogen bonds. And we are talking about how heterogeneous this grid is (for example, in density). The idea of water as hydrogen-bonded clusters floating in a sea of unbonded water molecules was put to an end in the early eighties, when G. Stanley applied the percolation theory, which describes the phase transitions of water, to the water model.
In 1999, the famous Russian water researcher S.V. Zenin defended his doctoral dissertation at the Institute of Medical and Biological Problems of the Russian Academy of Sciences on cluster theory, which was a significant step in the advancement of this area of research, the complexity of which is enhanced by the fact that they are at the intersection of three sciences: physics, chemistry and biology. Based on data obtained by three physicochemical methods: refractometry (S.V. Zenin, B.V. Tyaglov, 1994), high-performance liquid chromatography (S.V. Zenin et al., 1998) and proton magnetic resonance (C S.V. Zenin, 1993) constructed and proved a geometric model of the main stable structural formation of water molecules (structured water), and then (S.V. Zenin, 2004) an image of these structures was obtained using a contrast-phase microscope.
 Science has now proven that the peculiarities of the physical properties of water and numerous short-lived hydrogen bonds between neighboring hydrogen and oxygen atoms in a water molecule create favorable opportunities for the formation of special associated structures (clusters) that perceive, store and transmit a wide variety of information.
Science has now proven that the peculiarities of the physical properties of water and numerous short-lived hydrogen bonds between neighboring hydrogen and oxygen atoms in a water molecule create favorable opportunities for the formation of special associated structures (clusters) that perceive, store and transmit a wide variety of information.
The structural unit of such water is a cluster consisting of clathrates, the nature of which is determined by long-range Coulomb forces. The structure of the clusters encodes information about the interactions that took place with these water molecules. In water clusters, due to the interaction between covalent and hydrogen bonds between oxygen atoms and hydrogen atoms, migration of a proton (H+) can occur via a relay mechanism, leading to delocalization of the proton within the cluster.
Water, consisting of many clusters of various types, forms a hierarchical spatial liquid crystal structure that can perceive and store huge amounts of information.
The figure (V.L. Voeikov) shows diagrams of several simple cluster structures as an example.
Some possible structures of water clusters

Physical fields of very different nature can be carriers of information. Thus, the possibility of remote information interaction of the liquid crystalline structure of water with objects of various natures using electromagnetic, acoustic and other fields has been established. The influencing object can also be a person.
Water is a source of ultra-weak and weak alternating electromagnetic radiation. The least chaotic electromagnetic radiation is created by structured water. In this case, the induction of a corresponding electromagnetic field may occur, changing the structural and information characteristics of biological objects.
In recent years, important data have been obtained on the properties of supercooled water. Studying water at low temperatures is very interesting, since it can be supercooled more than other liquids. Crystallization of water, as a rule, begins on some inhomogeneities either on the walls of the vessel or on floating particles of solid impurities. Therefore, it is not easy to find the temperature at which supercooled water would spontaneously crystallize. But scientists managed to do this, and now the temperature of the so-called homogeneous nucleation, when the formation of ice crystals occurs simultaneously throughout the entire volume, is known for pressures up to 0.3 GPa, that is, covering the regions of existence of ice II.
From atmospheric pressure to the boundary separating ices I and II, this temperature drops from 231 to 180 K, and then increases slightly to 190 K. Below this critical temperature, liquid water is impossible in principle.
Ice structure (picture on the right)
 However, there is a mystery associated with this temperature. In the mid-eighties, a new modification of amorphous ice was discovered - high-density ice, and this helped revive the idea of water as a mixture of two states. Not crystalline structures, but structures of amorphous ice of different densities were considered as prototypes. This concept was formulated in the most clear form by E.G. Ponyatovsky and V.V. Sinitsin, who wrote in 1999: “Water is considered as a regular solution of two components, the local configurations in which correspond to the short-range order of modifications of amorphous ice.” Moreover, by studying short-range order in supercooled water at high pressure using neutron diffraction methods, scientists were able to find components corresponding to these structures.
However, there is a mystery associated with this temperature. In the mid-eighties, a new modification of amorphous ice was discovered - high-density ice, and this helped revive the idea of water as a mixture of two states. Not crystalline structures, but structures of amorphous ice of different densities were considered as prototypes. This concept was formulated in the most clear form by E.G. Ponyatovsky and V.V. Sinitsin, who wrote in 1999: “Water is considered as a regular solution of two components, the local configurations in which correspond to the short-range order of modifications of amorphous ice.” Moreover, by studying short-range order in supercooled water at high pressure using neutron diffraction methods, scientists were able to find components corresponding to these structures.
A consequence of the polymorphism of amorphous ice has also led to assumptions about the separation of water into two immiscible components at temperatures below the hypothetical low-temperature critical point. Unfortunately, according to researchers, this temperature at a pressure of 0.017 GPa is 230 K below the nucleation temperature, so no one has yet been able to observe the stratification of liquid water. Thus, the revival of the two-state model raised the question of the heterogeneity of the network of hydrogen bonds in liquid water. This heterogeneity can only be understood using computer modeling.
Speaking about the crystalline structure of water, it should be noted that 14 modifications of ice are known, most of which are not found in nature, in which water molecules both retain their individuality and are connected by hydrogen bonds. On the other hand, there are many variants of the hydrogen bond network in clathrate hydrates. The energies of these networks (high-pressure ices and clathrate hydrates) are not much higher than the energies of cubic and hexagonal ices. Therefore, fragments of such structures can also appear in liquid water. It is possible to construct countless different non-periodic fragments, the molecules of which have four nearest neighbors located approximately at the vertices of the tetrahedron, but their structure does not correspond to the structures of known modifications of ice. As numerous calculations have shown, the interaction energies of molecules in such fragments will be close to each other, and there is no reason to say that any structure should prevail in liquid water.
Structural studies of water can be studied using different methods; proton magnetic resonance spectroscopy, infrared spectroscopy, X-ray diffraction, etc. For example, the diffraction of X-rays and neutrons has been studied many times. However, these experiments cannot provide detailed information about the structure. Inhomogeneities that differ in density could be seen by the scattering of X-rays and neutrons at small angles, but such inhomogeneities must be large, consisting of hundreds of water molecules. It would be possible to see them by studying the scattering of light. However, water is an exceptionally clear liquid. The only result of diffraction experiments is the radial distribution function, that is, the distance between the atoms of oxygen, hydrogen and oxygen-hydrogen. It is clear from them that there is no long-range order in the arrangement of water molecules. These functions decay much faster for water than for most other liquids. For example, the distribution of distances between oxygen atoms at temperatures close to room temperature gives only three maxima, at 2.8, 4.5 and 6.7. The first maximum corresponds to the distance to the nearest neighbors, and its value is approximately equal to the length of the hydrogen bond. The second maximum is close to the average length of a tetrahedron edge: remember that water molecules in hexagonal ice are located along the vertices of a tetrahedron described around the central molecule. And the third maximum, very weakly expressed, corresponds to the distance to third and more distant neighbors in the hydrogen network. This maximum itself is not very bright, and there is no need to talk about further peaks. There have been attempts to obtain more detailed information from these distributions. So in 1969, I.S. Andrianov and I.Z. Fisher found the distances up to the eighth neighbor, while to the fifth neighbor it turned out to be 3, and to the sixth 3.1. This makes it possible to obtain data on the distant environment of water molecules.
Another method of studying the structure - neutron diffraction on water crystals - is carried out in exactly the same way as x-ray diffraction. However, due to the fact that the neutron scattering lengths do not differ so much between different atoms, the isomorphic substitution method becomes unacceptable. In practice, one usually works with a crystal whose molecular structure has already been approximately determined by other methods. Neutron diffraction intensities are then measured for this crystal. Based on these results, a Fourier transform is performed, during which the measured neutron intensities and phases are used, calculated taking into account non-hydrogen atoms, i.e. oxygen atoms, the position of which in the structure model is known. Then, on the Fourier map obtained in this way, the hydrogen and deuterium atoms are represented with much larger weights than on the electron density map, because the contribution of these atoms to neutron scattering is very large. Using this density map, you can, for example, determine the positions of hydrogen atoms (negative density) and deuterium (positive density).
A variation of this method is possible, which consists in the fact that the crystal formed in water is kept in heavy water before measurements. In this case, neutron diffraction not only makes it possible to determine where hydrogen atoms are located, but also identifies those of them that can be exchanged for deuterium, which is especially important when studying isotope (H-D) exchange. Such information helps to confirm that the structure has been established correctly.
Other methods also make it possible to study the dynamics of water molecules. These are experiments on quasi-elastic neutron scattering, ultrafast IR spectroscopy and the study of water diffusion using NMR or labeled deuterium atoms. The NMR spectroscopy method is based on the fact that the nucleus of a hydrogen atom has a magnetic moment—spin—that interacts with magnetic fields, constant and variable. From the NMR spectrum one can judge in what environment these atoms and nuclei are located, thus obtaining information about the structure of the molecule.
As a result of experiments on quasi-elastic neutron scattering in water crystals, the most important parameter was measured - the self-diffusion coefficient at various pressures and temperatures. To judge the self-diffusion coefficient from quasielastic neutron scattering, it is necessary to make an assumption about the nature of the molecular motion. If they move in accordance with the model of Ya.I. Frenkel (a famous Russian theoretical physicist, author of the “Kinetic Theory of Liquids” - a classic book translated into many languages), also called the “jump-waiting” model, then the time of settled life (the time between jumping) of a molecule is 3.2 picoseconds. The latest methods of femtosecond laser spectroscopy have made it possible to estimate the lifetime of a broken hydrogen bond: it takes a proton 200 fs to find a partner. However, these are all average values. It is possible to study the details of the structure and nature of the movement of water molecules only with the help of computer simulation, sometimes called a numerical experiment.

This is what the structure of water looks like according to the results of computer modeling (according to Doctor of Chemical Sciences G.G. Malenkov). The general disordered structure can be divided into two types of regions (shown as dark and light balls), which differ in their structure, for example, in the volume of the Voronoi polyhedron (a), the degree of tetrahedrality of the immediate environment (b), the value of potential energy (c), and also in the presence of four hydrogen bonds in each molecule (d). However, these areas literally in a moment, after a few picoseconds, will change their location.
The simulation is carried out like this. The ice structure is taken and heated until it melts. Then, after some time for the water to forget about its crystalline origin, instantaneous microphotographs are taken.
To analyze the structure of water, three parameters are selected:
- degree of deviation of the local environment of the molecule from the vertices of a regular tetrahedron;
-potential energy of molecules;
-the volume of the so-called Voronoi polyhedron.
To construct this polyhedron, take an edge from a given molecule to the nearest one, divide it in half, and draw a plane through this point perpendicular to the edge. This gives the volume per molecule. The volume of a polyhedron is density, tetrahedrality is the degree of distortion of hydrogen bonds, energy is the degree of stability of the molecular configuration. Molecules with similar values of each of these parameters tend to group together into separate clusters. Both low-density and high-density regions have different energy values, but they can also have the same energy values. Experiments have shown that areas with different structures, clusters arise spontaneously and spontaneously disintegrate. The entire structure of water is alive and constantly changing, and the time during which these changes occur is very short. The researchers monitored the movements of the molecules and found that they performed irregular vibrations with a frequency of about 0.5 ps and an amplitude of 1 angstrom. Rare slow jumps of angstroms that last for picoseconds were also observed. In general, in 30 ps a molecule can move 8-10 angstroms. The lifetime of the local environment is also short. Regions composed of molecules with similar volume values of the Voronoi polyhedron can decay in 0.5 ps, or they can live for several picoseconds. But the distribution of hydrogen bond lifetimes is very large. But this time does not exceed 40 ps, and the average value is several ps.
In conclusion, it should be emphasized that The theory of the cluster structure of water has many pitfalls. For example, Zenin suggests that the main structural element of water is a cluster of 57 molecules formed by the fusion of four dodecahedrons. They have common faces, and their centers form a regular tetrahedron. It has long been known that water molecules can be located at the vertices of a pentagonal dodecahedron; Such a dodecahedron is the basis of gas hydrates. Therefore, there is nothing surprising in the assumption of the existence of such structures in water, although it has already been said that no specific structure can be predominant and exist for a long time. It is therefore strange that this element is assumed to be the main one and that it contains exactly 57 molecules. From balls, for example, you can assemble the same structures, which consist of dodecahedrons adjacent to each other and contain 200 molecules. Zenin claims that the process of three-dimensional polymerization of water stops at 57 molecules. In his opinion, there should not be larger associates. However, if this were so, hexagonal ice crystals, which contain a huge number of molecules linked together by hydrogen bonds, could not precipitate from water vapor. It is not at all clear why the growth of the Zenin cluster stopped at 57 molecules. To avoid contradictions, Zenin packs clusters into more complex formations—rhombohedra—of almost a thousand molecules, and the original clusters do not form hydrogen bonds with each other. Why? How are the molecules on their surface different from those inside? According to Zenin, the pattern of hydroxyl groups on the surface of rhombohedrons provides the memory of water. Consequently, the water molecules in these large complexes are rigidly fixed, and the complexes themselves are solids. Such water will not flow, and its melting point, which is related to molecular weight, should be very high.
What properties of water does Zenin's model explain? Since the model is based on tetrahedral structures, it can be more or less consistent with X-ray and neutron diffraction data. However, it is unlikely that the model can explain the decrease in density during melting; the packing of dodecahedrons is less dense than ice. But it is most difficult to agree with a model with dynamic properties - fluidity, a large value of the self-diffusion coefficient, short correlation and dielectric relaxation times, which are measured in picoseconds.
Ph.D. O.V. Mosin
References:
G.G. Malenkov. Advances in Physical Chemistry, 2001
S.V.Zenin, B.M. Polanuer, B.V. Tyaglov. Experimental proof of the presence of water fractions. G. Homeopathic medicine and acupuncture. 1997.No.2.P.42-46.
S.V. Zenin, B.V. Tyaglov. Hydrophobic model of the structure of associates of water molecules. J. Physical Chemistry. 1994. T. 68. No. 4. P. 636-641.
S.V. Zenin Study of the structure of water using the proton magnetic resonance method. Dokl.RAN.1993.T.332.No.3.S.328-329.
S.V.Zenin, B.V.Tyaglov. The nature of hydrophobic interaction. The emergence of orientation fields in aqueous solutions. J. Physical Chemistry. 1994. T. 68. No. 3. P. 500-503.
S.V. Zenin, B.V. Tyaglov, G.B. Sergeev, Z.A. Shabarova. Study of intramolecular interactions in nucleotidamides using NMR. Materials of the 2nd All-Union Conf. By dynamic Stereochemistry. Odessa.1975.p.53.
S.V. Zenin. The structured state of water as the basis for controlling the behavior and safety of living systems. Thesis. Doctor of Biological Sciences. State Scientific Center "Institute of Medical and Biological Problems" (SSC "IMBP"). Protected 1999. 05. 27. UDC 577.32:57.089.001.66.207 p.
IN AND. Slesarev. Research progress report
Ice formation is always associated with the appearance of a phase interface. The work Lk expended in this case is spent mainly on overcoming the interphase surface tension of the primary nucleus of an ice crystal, the probability of the occurrence of which is determined by the laws of statistical physics.
The crystallization of water is usually characterized by two main factors associated with its supercooling: the rate of nucleation of crystallization centers wi and the linear crystallization rate o>2.
Viscous liquids with minimal values of W\ and Shr, even at a relatively low cooling rate, can be transferred to a solid amorphous (glassy) state, bypassing crystallization. Low-viscosity water with high values of W\ and w2 for such a transition requires a very high cooling rate (>4000°C/s) in order to “overshoot” the temperature zone of maximum coistallization.
According to Frenkel G112], even in an absolutely pure free liquid, if it is sufficiently supercooled, crystal nuclei of a critical size can arise due to fluctuations, which, under favorable conditions, become crystallization centers. For crystallization to develop, it is necessary that the number of crystals being formed exceed the number of crystals being destroyed. The assumption that water in the pre-crystallization state contains many nuclei of the solid phase is to a certain extent confirmed, for example, by the anomalous increase in the speed of sound in water at a temperature of about 0 ° C.
Practically, the seeds of water crystallization are the minor solid impurities that are always present in it, which further reduce the interfacial surface tension and the work of crystallization Ak. To induce crystallization in supercooled water (and water vapor), microseeds made from ice or from a substance practically isomorphic to ice, for example, silver iodide (Agl), are most effective.
During the crystallization (and melting) of ice, an electrical potential difference always arises at the phase boundary as a result of partial polarization, and the strength toKa is set proportional to the rate of phase transformation. Crystallization of water bound, for example, by a capillary, requires preliminary restoration of the corresponding structure of water, including hydrogen bonds broken by the capillary.
In the usual case, intra-aqueous ice crystals formed in zones of sufficiently supercooled water, with symmetry of the medium and heat transfer, grow in the directions of their optical axes. In this case, crystal growth occurs in jumps and most vigorously at the vertices and edges, i.e., where there are more unsaturated bonds.
During the crystallization of water, which requires its supercooling, the temperature of the emerging phase - the embryo of an intra-aqueous ice crystal - is in principle equal to the phase transformation temperature of 0°C. Around the emerging ice crystal nuclei, due to the release of the heat of crystallization, a temperature jump occurs, local supercooling of the water is eliminated, and individual ice nuclei that have arisen can melt. Therefore, to maintain the ice formation process, continuous removal of the heat of crystallization is necessary. At 0°C, a dynamic equilibrium of ice and water can occur.
Surface ice crystallization process localized in the boundary layer of supercooled water. According to Costa, the supercooling of water during the formation of surface ice is a function of the linear rate of crystallization of water on the cooled surface and ranges from -0.02° to -0.11° C at rates from 2 to 30 mm/min. In this case, the temperature of the wetted ice surface should be below 0° C.
During crystallization, water turns into ice - a new, thermodynamically more stable phase. The reverse transformation of the substance also partially occurs, but the transition of molecules into the solid phase predominates. The restoration (according to Pople - straightening) of hydrogen bonds and other phenomena that occurs in the case of crystallization change the quartz-like structure of liquid water to a less dense structure of ice.
Since with the usual tridymite-like structure of ice, each of its molecules is associated with three molecules of its structural layer and one molecule of the neighboring layer, then the coordination number of molecules in ice is four. Changes in a number of physical properties of water during cooling and freezing clearly reflect transformations in its structure.
Thus, in the case of cooling water at a normal pressure of 0.101325 MPa from a temperature t = 4 ° C (277.15 K) to * = 0 ° C (273.15 K), its density drops from 1000 to 999.9 kg/ m3, and when converted into ice it further decreases to 916.8 kg/m3 (рл « «917(1-0.00015 t). According to calculations, the mass ratio of 1 mole of water and ice is 18.02: 19.66 «0.916.
During the crystallization of water, which requires the removal of specific heat hl = 334 kJ/kg, the heat capacity changes from sv = 4.23 to sv = 2.12 kJ/ (kg-K), and thermal conductivity from Rav = 0.55 to Rav53 = 2 .22 W/ (m K). Compared to water, ice has an average dielectric constant 30 times less, and electrical conductivity 500 times or more.
The anomalous drop in water density is caused mainly by a decrease in the compactness of the average arrangement of molecules. The features of water and ice, in particular, are explained by changes in the relative amounts of molecules with a temporarily fixed position and molecules moving, as well as the influence of hydrogen bonds, cavities in structures and polymerization of molecules.
Ice single crystals that appear during the crystallization of water do not have an ideal crystal lattice due to inevitable structural defects, in particular the type of dislocations (shears) caused by disruption of the packing of molecules and the alternation of atomic planes.
Thermal motion causes dislocation escape of individual microparticles into the interstices of crystal lattices and the formation of vacancies (“holes”) in the crystal structure, similar to the vacancies found in liquids, in particular in water. It is believed that dislocation defects are one of the reasons for the high plasticity of ice, on which the long-term strength of ice refrigerators depends. Ice usually crystallizes in a tridymite-like hexagonal system. However, at temperatures below -120° C, steam ice has a diamond-like cubic structure. At temperatures below -160° C and a high cooling rate, steam in a vacuum turns into glassy, almost amorphous ice with a density of 1300-2470 kg/m3. Single crystals of intra-aqueous and surface ice arise during supercooling from water molecules with minimal energy.
According to Altberg, natural intra-water (bottom) ice is formed in a river due to the convective transport of supercooled surface water into the flow and its subsequent crystallization mainly on grains of sand and other solid objects.
In the case of the formation of surface ice in a reservoir, individual ice single crystals that arise at atmospheric temperatures usually below 0°C unite, in particular, into needle-shaped horizontal crystals, which intersect as they grow and create a lattice. The gaps of the ice lattice are filled with single crystals, also united into crystallites, which complete the final stage of the formation of a continuous crust of polycrystalline ice, mainly with a chaotic arrangement of crystals. With strong nighttime heat radiation from the surface of calm water, an ice crust can form even at positive temperatures.
The further growth of crystals of the initial ice crust is influenced by neighboring crystals. In this case, due to the anisotropy of growth, there is a predominant development of crystals of two types: a) with vertical optical axes perpendicular to the ice formation surface - in calm water with a relatively large temperature gradient and b) with horizontal axes parallel to the ice formation surface - in moving water and its approximate isotherm.
Provided with nutrition, growing crystals exhibit the so-called crystallization force, which repels obstacles. With slow crystallization and good circulation of fresh water, most of the water impurities are pushed out and transparent ice of a greenish-blue hue is formed. Ice is formed mainly with regularly oriented large crystallites in the form of a prism with a diameter of the order of several millimeters and with a relatively small amount of impurities. With rapid crystallization and weak circulation of water, the ice turns out to be opaque, white (matte ice) and in this case is a body with a chaotic arrangement of intergrowths of small crystals, usually with a diameter of less than 1 mm, interspersed with solid, liquid and gaseous (air) impurities. During the rapid crystallization of water with an increased amount of impurities, they are sometimes located not only between the crystals, but also on the basal planes inside them. The layers between crystallites always contain much more impurities than the layers between single crystals. In the particular case of river ice, intercrystalline layers have a thickness of the order of 3 microns at a freezing temperature of -2° C to 0.3 microns at a temperature of about -20° C. It is noted that the size of ice crystals from water with an admixture of water-soluble salts is inversely proportional to the freezing rate and concentration salts
If ice does not form on a flat surface of water, but in very small water drops, present, for example, in clouds, where significant supercooling of water can occur (down to -40 ° C and below), then its crystallization may begin not from the outside, but from the inside drops where inland ice forms. Large drops of water after hypothermia usually begin to freeze outside.
When fresh water crystallizes, the growing ice front is almost smooth. In this case, water, containing about 40 g of air per ton at O9 C (at 30 ° C - only 20 g), during crystallization, when the front moves, it releases air into the extra- or intercrystalline space.
When salt water crystallizes (begins at a temperature determined by the composition and concentration of salts), the growing ice front is rough, with protrusions, the tops of which are located in zones of lowest salt concentration. Water that is less bound by hydration to salt ions crystallizes first. Subsequently, the salt ions can be dehydrated to one degree or another and the salts will fall out of solution in accordance with their solubility. In this case, crystalline hydrates corresponding to the temperature can be formed. In ice with water-soluble impurities, the latter are mainly located in cells of crystals, which is important, for example, in the production of brine ice.
When ice forms among other structures, their deformation usually occurs, in particular in the case of freezing of wet soil or water in a porous zerotor. The least deformation is ensured by rapid and uniform hardening of water in biological media with cryoprotectants (glycerin, etc.). In this case, one part of the water is “vitrified”, and the other binds or forms microcrystals, located mainly outside the biological cells. A special process is the crystallization of ice by sublimation from steam (and the reverse phenomenon of sublimation during the evaporation of ice).
For the operation of ice refrigerators, both the evaporation of ice barriers and the formation of sublimation ice in the form of a “snow coat” are important. At low enough temperatures, sublimated ice forms as snowflakes, for example in high clouds. Crystallization of atmospheric ice in the form of snow begins on seeds, in this case dust particles. The formation and growth of crystalline snowflakes, consisting of ordinary or sublimated ice, are related to the temperature, pressure and humidity of the atmosphere. Only large snowflakes that have crystallized and reached a critical mass descend to the ground.
It should be noted that the growth of large snowflakes at the expense of small crystals and drops is associated with increased water vapor pressure for small crystals and drops. The elasticity of vapor depends on the curvature and surface tension of water droplets or ice crystals. The artificial introduction of ice formation seeds into clouds has already been practically used in the Dnieper region for snowing winter crops during winters with little snow.
Ice melting. Ice formation is preceded by one or another supercooling of water, and melting is a pre-melting process that is practically not associated with overheating of the solid phase, since from the surface ice at normal pressure begins to melt at a temperature (GS (273.15 K). During melting, unlike crystallization, it does not the significant force of surface tension of water is overcome.The long-range order of the arrangement of molecules, inherent in ice, changes during melting to the short-range order, characteristic of water.
Internal energy increases when ice melts. Based on the specific heat of melting of ice 334 kJ/kg and the heat of sublimation 2840 kJ/kg, which characterizes the breaking of all molecular bonds, the degree of weakening of molecular bonds during melting can be taken equal to 12%. Of these, approximately 9% are hydrogen bonds and only 3% are van der Waals bonds.
When ice melts, the length of time the molecules remain in the equilibrium position changes dramatically. The activation energy (potential barrier) E decreases, since the E of water is less than the E of ice. Always present defects in the crystal lattice structure and impurities further reduce the activation energy. Melting of ice usually begins from its surface, on the faces and edges of crystals, as well as in the locations of impurities, which are the seeds of melting. The surface of melting ice is always micro-rough.
The most complicated process is the melting of ice within other structures, for example in the case of icy soil. Water-soluble salts in ice help it melt both outside and inside.
It must be emphasized that fresh ice melt temporarily retains some physical features that are closer to ice than to near-zero temperature water. The molecular properties inherent in ice are temporarily transferred to melt water, which apparently “determines its increased biological activity. Electrical processes during ice melting, as well as the special activity of ice and freshly melted water can affect, for example, food products cooled by melting ice. Technologically, it is also important that melting ice absorbs many gases well, and therefore odors.
The physics and chemistry of water and ice are discussed in more detail in the monographs of Fritzman, Dorsey and Fletcher, especially the melting process in the work of Ubbelohde, the structure of water and ice in the works of Shumsky, Zatsepina, Eisenberg and Kautzman.
Properties of water
Why is water water?
Among the vast variety of substances, water with its physical and chemical properties occupies a very special, exceptional place. And this must be taken literally.
Almost all physical and chemical properties of water are exceptions in nature. It truly is the most amazing substance in the world. Water is amazing not only for the variety of isotopic forms of the molecule and not only for the hopes that are associated with it as an inexhaustible source of energy for the future. In addition, it is amazing for its very ordinary properties.
How is a water molecule built?
How one molecule of water is built is now known very precisely. It's built like this.
 The relative positions of the nuclei of hydrogen and oxygen atoms and the distance between them have been well studied and measured. It turned out that the water molecule is nonlinear. Together with the electron shells of the atoms, a water molecule, if you look at it “from the side,” could be depicted like this:
The relative positions of the nuclei of hydrogen and oxygen atoms and the distance between them have been well studied and measured. It turned out that the water molecule is nonlinear. Together with the electron shells of the atoms, a water molecule, if you look at it “from the side,” could be depicted like this:
that is, geometrically, the mutual arrangement of charges in a molecule can be depicted as a simple tetrahedron. All water molecules with any isotopic composition are built exactly the same.
How many water molecules are there in the ocean?
One. And this answer is not exactly a joke. Of course, anyone can, by looking at a reference book and finding out how much water there is in the World Ocean, easily calculate how many H2O molecules it contains. But such an answer will not be entirely correct. Water is a special substance. Due to their unique structure, individual molecules interact with each other. A special chemical bond arises due to the fact that each of the hydrogen atoms of one molecule attracts electrons of oxygen atoms in neighboring molecules. Due to this hydrogen bond, each water molecule becomes quite tightly bound to four other neighboring molecules, just as shown in the diagram. True, this diagram is too simplified - it is flat, otherwise it cannot be depicted in the figure. Let's imagine a slightly more accurate picture. To do this, you need to take into account that the plane in which hydrogen bonds are located (they are indicated by a dotted line) in a water molecule is directed perpendicular to the plane of location of the hydrogen atoms.
All individual H2O molecules in water turn out to be connected into a single continuous spatial network - into one giant molecule. Therefore, the assertion of some physical chemists that the entire ocean is one molecule is quite justified. But this statement should not be taken too literally. Although all water molecules in water are connected to each other by hydrogen bonds, they are at the same time in a very complex mobile equilibrium, preserving the individual properties of individual molecules and forming complex aggregates. This idea applies not only to water: a piece of diamond is also one molecule.
How is an ice molecule built?
There are no special ice molecules. The molecules of water, due to their remarkable structure, are connected to each other in a piece of ice so that each of them is connected and surrounded by four other molecules. This leads to the appearance of a very loose ice structure, in which a lot of free volume remains. The correct crystalline structure of ice is expressed in the amazing grace of snowflakes and the beauty of frosty patterns on frozen window panes.
How are water molecules in water built?
Unfortunately, this very important issue has not yet been sufficiently studied. The structure of molecules in liquid water is very complex. When ice melts, its network structure is partially preserved in the resulting water. The molecules in melt water consist of many simple molecules - aggregates that retain the properties of ice. As the temperature rises, some of them disintegrate and their sizes become smaller.
Mutual attraction leads to the fact that the average size of a complex water molecule in liquid water significantly exceeds the size of a single water molecule. This extraordinary molecular structure of water determines its extraordinary physicochemical properties.
What should the density of water be?
Isn't that a very strange question? Remember how the unit of mass was established - one gram. This is the mass of one cubic centimeter of water. This means that there can be no doubt that the density of water should only be what it is. Can there be any doubt about this? Can. Theorists have calculated that if water did not retain a loose, ice-like structure in the liquid state and its molecules were tightly packed, then the density of water would be much higher. At 25°C it would be equal not to 1.0, but to 1.8 g/cm3.
At what temperature should water boil?
This question is also, of course, strange. After all, water boils at one hundred degrees. Everyone knows this. Moreover, everyone knows that it is the boiling point of water at normal atmospheric pressure that was chosen as one of the reference points of the temperature scale, conventionally designated 100°C.
However, the question is posed differently: at what temperature should water boil? After all, the boiling temperatures of various substances are not random. They depend on the position of the elements that make up their molecules in Mendeleev’s periodic table.
If we compare chemical compounds of different elements with the same composition that belong to the same group of the periodic table, it is easy to notice that the lower the atomic number of an element, the lower its atomic weight, the lower the boiling point of its compounds. Based on its chemical composition, water can be called an oxygen hydride. H2Te, H2Se and H2S are chemical analogues of water. If you monitor their boiling points and compare how the boiling points of hydrides change in other groups of the periodic table, then you can quite accurately determine the boiling point of any hydride, just like any other compound. Mendeleev himself was able to predict the properties of chemical compounds of elements not yet discovered in this way.
If we determine the boiling point of oxygen hydride by its position in the periodic table, it turns out that water should boil at -80 ° C. Consequently, water boils approximately one hundred and eighty degrees higher , than it should boil. The boiling point of water - this is its most common property - turns out to be extraordinary and surprising.
The properties of any chemical compound depend on the nature of the elements that form it and, therefore, on their position in Mendeleev’s periodic table of chemical elements. These graphs show the dependences of the boiling and melting temperatures of hydrogen compounds of groups IV and VI of the periodic system. Water is a striking exception. Due to the very small radius of the proton, the interaction forces between its molecules are so great that it is very difficult to separate them, which is why water boils and melts at abnormally high temperatures.

Graph A. Normal dependence of the boiling point of hydrides of group IV elements on their position in the periodic table.
Graph B. Among the hydrides of group VI elements, water has anomalous properties: water should boil at minus 80 - minus 90 ° C, but it boils at plus 100 ° C.

Graph B. Normal dependence of the melting temperature of hydrides of group IV elements on their position in the periodic table.
Graph D. Among the hydrides of group VI elements, water violates the order: it should melt at minus 100 ° C, and ice icicles melt at 0 ° C.
At what temperature does water freeze?
Isn't it true that the question is no less strange than the previous ones? Well, who doesn’t know that water freezes at zero degrees? This is the second reference point of the thermometer. This is the most common property of water. But even in this case, one can ask: at what temperature should water freeze in accordance with its chemical nature? It turns out that oxygen hydride, based on its position in the periodic table, should have solidified at one hundred degrees below zero.
How many liquid states of water are there?
This question is not so easy to answer. Of course, there is also one thing - the liquid water we are all familiar with. But liquid water has such extraordinary properties that one has to wonder whether such a simple, seemingly non-provoking
no doubt the answer? Water is the only substance in the world that, after melting, first contracts and then begins to expand as the temperature rises. At approximately 4°C, water is at its highest density. This rare anomaly in the properties of water is explained by the fact that in reality liquid water is a complex solution of a completely unusual composition: it is a solution of water in water.
When ice melts, large, complex water molecules are first formed. They retain remnants of the loose crystalline structure of ice and are dissolved in ordinary low-molecular-weight water. Therefore, at first the density of water is low, but as the temperature increases, these large molecules break down and so the density of the water increases until normal thermal expansion takes over, at which point the density of the water falls again. If this is true, then several states of water are possible, but no one knows how to separate them. And it is still unknown whether this will ever be possible. This extraordinary property of water is of great importance for life. In reservoirs, before the onset of winter, the cooling water gradually drops down until the temperature of the entire reservoir reaches 4°C. With further cooling, the colder water remains on top and all mixing stops. As a result, an extraordinary situation is created: a thin layer of cold water becomes like a “warm blanket” for all the inhabitants of the underwater world. At 4°C they clearly feel quite well.
What should be easier - water or ice?
Who doesn’t know this... After all, ice floats on water. Giant icebergs float in the ocean. Lakes in winter are covered with a floating continuous layer of ice. Of course, ice is lighter than water.
But why "of course"? Is it that clear? On the contrary, the volume of all solids increases during melting, and they drown in their own melt. But ice floats in water. This property of water is an anomaly in nature, an exception, and, moreover, an absolutely remarkable exception.

The positive charges in a water molecule are associated with hydrogen atoms. The negative charges are the valence electrons of oxygen. Their relative arrangement in a water molecule can be depicted as a simple tetrahedron.
Let's try to imagine what the world would look like if water had normal properties and ice was, as any normal substance should be, denser than liquid water. In winter, denser ice freezing from above would sink into the water, continuously sinking to the bottom of the reservoir. In summer, the ice, protected by a layer of cold water, could not melt. Gradually, all lakes, ponds, rivers, streams would freeze completely, turning into giant blocks of ice. Finally, the seas would freeze, followed by the oceans. Our beautiful, blooming green world would become a continuous icy desert, covered here and there with a thin layer of melt water.
How many ices are there?
In nature on our Earth there is only one: ordinary ice. Ice is a rock with extraordinary properties. It is solid, but flows like a liquid, and there are huge rivers of ice that flow slowly down from the high mountains. Ice is changeable - it continuously disappears and forms again. Ice is unusually strong and durable - for tens of thousands of years it preserves without changes the bodies of mammoths that accidentally died in glacial cracks. In his laboratories, man managed to discover at least six more different, no less amazing ices. They cannot be found in nature. They can only exist at very high pressures. Ordinary ice is preserved up to a pressure of 208 MPa (megapascals), but at this pressure it melts at - 22 °C. If the pressure is higher than 208 MPa, dense ice appears - ice-III. It is heavier than water and sinks in it. At a lower temperature and higher pressure - up to 300 MPa - even denser ice-P is formed. Pressure above 500 MPa turns ice into ice-V. This ice can be heated to almost 0 ° C, and it will not melt, although it is under enormous pressure. At a pressure of about 2 GPa (gigapascals), ice-VI appears. This is literally hot ice - it can withstand temperatures of 80° C without melting. Ice-VII, found at 3GP pressure, can perhaps be called hot ice. This is the densest and most refractory ice known. It only melts at 190° above zero.
Ice-VII has an unusually high hardness. This ice can even cause sudden disasters. The bearings in which the shafts of powerful power plant turbines rotate develop enormous pressure. If even a little water gets into the grease, it will freeze, even though the bearing temperature is very high. The resulting ice-VII particles, which have enormous hardness, will begin to destroy the shaft and bearing and quickly cause them to fail.
Maybe there is ice in space too?
As if there is, and at the same time very strange. But scientists on Earth discovered it, although such ice cannot exist on our planet. The density of all currently known ice, even at very high pressures, only very slightly exceeds 1 g/cm3. The density of the hexagonal and cubic modifications of ice at very low pressures and temperatures, even close to absolute zero, is slightly less than unity. Their density is 0.94 g/cm3.
But it turned out that in a vacuum, at negligible pressures and at temperatures below -170 ° C, under conditions when the formation of ice occurs when it condenses from steam on a cooled solid surface, absolutely amazing ice appears. Its density is... 2.3 g/cm3. All ice known so far is crystalline, but this new ice is apparently amorphous, characterized by a random relative arrangement of individual water molecules; It does not have a specific crystal structure. For this reason, it is sometimes called glass ice. Scientists are confident that this amazing ice must arise in space conditions and play a big role in the physics of planets and comets. The discovery of such super-dense ice was unexpected for physicists.
What does it take for the ice to melt?
A lot of heat. Much more than it would take to melt the same amount of any other substance. The exceptionally high specific heat of fusion -80 cal (335 J) per gram of ice is also an anomalous property of water. When water freezes, the same amount of heat is released again.
When winter comes, ice forms, snow falls and water gives back heat, warming the ground and air. They resist the cold and soften the transition to harsh winter. Thanks to this wonderful property of water, autumn and spring exist on our planet.
How much heat is needed to heat water?
So many. More than it takes to heat an equal amount of any other substance. It takes one calorie (4.2 J) to heat a gram of water one degree. This is more than double the heat capacity of any chemical compound.
Water is a substance that is extraordinary in its most ordinary properties for us. Of course, this ability of water is very important not only when cooking dinner in the kitchen. Water is the great distributor of heat throughout the Earth. Heated by the Sun under the equator, it transfers heat in the World Ocean with giant streams of sea currents to the distant polar regions, where life is possible only thanks to this amazing feature of water.
Why is the water in the sea salty?
This is perhaps one of the most important consequences of one of the most amazing properties of water. In its molecule, the centers of positive and negative charges are strongly displaced relative to each other. Therefore, water has an exceptionally high, anomalous value of dielectric constant. For water, e = 80, and for air and vacuum, e = 1. This means that any two opposite charges in water are mutually attracted to each other with a force 80 times less than in air. After all, according to Coulomb's law:
![]()
But still, intermolecular bonds in all bodies, which determine the strength of the body, are caused by the interaction between the positive charges of atomic nuclei and negative electrons. On the surface of a body immersed in water, the forces acting between molecules or atoms are weakened under the influence of water by almost a hundred times. If the remaining bond strength between molecules becomes insufficient to withstand the effects of thermal motion, molecules or atoms of the body begin to break away from its surface and pass into water. The body begins to dissolve, breaking up either into individual molecules, like sugar in a glass of tea, or into charged particles - ions, like table salt.
It is thanks to its abnormally high dielectric constant that water is one of the most powerful solvents. It is even capable of dissolving any rock on the earth's surface. Slowly and inevitably, it destroys even granites, leaching easily soluble components from them.
Streams, rivers and rivers carry impurities dissolved in water into the ocean. The water from the ocean evaporates and returns to the earth again to continue its eternal work again and again. And dissolved salts remain in the seas and oceans.
Do not think that water dissolves and carries into the sea only what is easily soluble, and that sea water contains only ordinary salt that stands on the dinner table. No, sea water contains almost all the elements that exist in nature. It contains magnesium, calcium, sulfur, bromine, iodine, and fluorine. Iron, copper, nickel, tin, uranium, cobalt, even silver and gold were found in it in smaller quantities. Chemists found over sixty elements in sea water. Probably all the others will be found as well. Most of the salt in sea water is table salt. That's why the water in the sea is salty.
Is it possible to run on the surface of water?
Can. To see this, look at the surface of any pond or lake in summer. A lot of living and fast people not only walk on water, but also run. If we consider that the support area of the legs of these insects is very small, then it is not difficult to understand that, despite their low weight, the surface of the water can withstand significant pressure without breaking through.
Can water flow upward?
Yes maybe. This happens all the time and everywhere. The water itself rises up in the soil, wetting the entire thickness of the earth from the groundwater level. The water itself rises up through the capillary vessels of the tree and helps the plant deliver dissolved nutrients to great heights - from the roots deeply hidden in the ground to the leaves and fruits. The water itself moves upward in the pores of the blotting paper when you have to dry a blot, or in the fabric of a towel when you wipe your face. In very thin tubes - in capillaries - water can rise to a height of several meters.
What explains this?
Another remarkable feature of water is its exceptionally high surface tension. Water molecules on its surface experience the forces of intermolecular attraction only on one side, and in water this interaction is anomalously strong. Therefore, every molecule on its surface is drawn into the liquid. As a result, a force arises that tightens the surface of the liquid. In water it is especially strong: its surface tension is 72 mN/m (millinewtons per meter).
Can water remember?
This question sounds, admittedly, very unusual, but it is quite serious and very important. It concerns a large physico-chemical problem, which in its most important part has not yet been investigated. This question has just been posed in science, but it has not yet found an answer to it.
The question is: does the previous history of water influence its physical and chemical properties and is it possible, by studying the properties of water, to find out what happened to it earlier - to make the water itself “remember” and tell us about it. Yes, perhaps, as surprising as it may seem. The easiest way to understand this is with a simple, but very interesting and extraordinary example - the memory of ice.
Ice is water after all. When water evaporates, the isotopic composition of water and steam changes. Light water evaporates, although to an insignificant extent, faster than heavy water.
When natural water evaporates, the composition changes in the isotopic content of not only deuterium, but also heavy oxygen. These changes in the isotopic composition of steam have been very well studied, and their dependence on temperature has also been well studied.
Recently, scientists performed a remarkable experiment. In the Arctic, in the thickness of a huge glacier in northern Greenland, a borehole was sunk and a giant ice core almost one and a half kilometers long was drilled and extracted. The annual layers of growing ice were clearly visible on it. Along the entire length of the core, these layers were subjected to isotopic analysis, and based on the relative content of heavy isotopes of hydrogen and oxygen - deuterium and 18O - the formation temperatures of annual ice layers in each core section were determined. The date of formation of the annual layer was determined by direct counting. In this way, the climate situation on Earth was restored for a millennium. Water managed to remember and record all this in the deep layers of the Greenland glacier.
As a result of isotopic analyzes of ice layers, scientists constructed a climate change curve on Earth. It turned out that our average temperature is subject to secular fluctuations. It was very cold in the 15th century, at the end of the 17th century. and at the beginning of the 19th century. The hottest years were 1550 and 1930.
Then what is the mystery of the “memory” of water?
The fact is that in recent years, science has gradually accumulated many amazing and completely incomprehensible facts. Some of them are firmly established, others require quantitative reliable confirmation, and all of them are still waiting to be explained.
For example, no one yet knows what happens to water flowing through a strong magnetic field. Theoretical physicists are absolutely sure that nothing can and will not happen to it, reinforcing their conviction with completely reliable theoretical calculations, from which it follows that after the cessation of the magnetic field, the water should instantly return to its previous state and remain as it was . And experience shows that it changes and becomes different.
Is there a big difference? Judge for yourself. From ordinary water in a steam boiler, dissolved salts, released, are deposited in a dense and rock-hard layer on the walls of the boiler pipes, and from magnetized water (as it is now called in technology) they fall out in the form of a loose sediment suspended in the water. It seems like the difference is small. But it depends on the point of view. According to workers at thermal power plants, this difference is extremely significant, since magnetized water ensures normal and uninterrupted operation of giant power plants: the walls of steam boiler pipes do not become overgrown, heat transfer is higher, and electricity generation is higher. Magnetic water treatment has long been installed at many thermal stations, but neither engineers nor scientists know how and why it works. In addition, it has been observed experimentally that after magnetic treatment of water, the processes of crystallization, dissolution, adsorption are accelerated in it, and wetting changes... however, in all cases the effects are small and difficult to reproduce.
The effect of a magnetic field on water (necessarily fast-flowing) lasts for small fractions of a second, but the water “remembers” this for tens of hours. Why is unknown. In this matter, practice is far ahead of science. After all, it is further unknown what exactly magnetic treatment affects - water or the impurities contained in it. There is no such thing as pure water.
The “memory” of water is not limited to the preservation of the effects of magnetic influence. In science, many facts and observations exist and are gradually accumulating, showing that water seems to “remember” that it was previously frozen.
Melt water, recently formed by melting a piece of ice, also seems to be different from the water from which this piece of ice was formed. In melt water, seeds germinate faster and better, sprouts develop faster; further, chickens that receive melt water seem to grow and develop faster. In addition to the amazing properties of melt water, established by biologists, purely physical and chemical differences are also known, for example, melt water differs in viscosity and dielectric constant. The viscosity of melt water takes on its usual value for water only 3-6 days after melting. Why this is so (if it is so), no one else knows.
Most researchers call this area of phenomena the “structural memory” of water, believing that all these strange manifestations of the influence of the previous history of water on its properties are explained by changes in the fine structure of its molecular state. Maybe this is so, but... to name it does not mean to explain it. There is still an important problem in science: why and how water “remembers” what happened to it.
Where did water come from on Earth?
Streams of cosmic rays - streams of particles with enormous energy - are forever permeating the Universe in all directions. Most of them contain protons - the nuclei of hydrogen atoms. In its movement in space, our planet is continuously subjected to “proton bombardment.” Penetrating the upper layers of the earth's atmosphere, protons capture electrons, turn into hydrogen atoms and immediately react with oxygen to form water. Calculations show that every year almost one and a half tons of such “cosmic” water is born in the stratosphere. At high altitudes at low temperatures, the elasticity of water vapor is very small and water molecules, gradually accumulating, condense on cosmic dust particles, forming mysterious noctilucent clouds. Scientists suggest that they consist of tiny ice crystals that arose from such “cosmic” water. Calculations showed that the water that appeared on Earth in this way throughout its history would be just enough to give birth to all the oceans of our planet. So, water came to Earth from space? But...
Geochemists do not consider water a heavenly guest. They are convinced that she is of earthly origin. The rocks that make up the earth's mantle, which lies between the central core of the Earth and the earth's crust, melted in places under the influence of the accumulating heat of radioactive decay of isotopes. Of these, volatile components were released: nitrogen, chlorine, carbon and sulfur compounds, and most of all water vapor was released.
How much could all volcanoes emit during eruptions during the entire existence of our planet?
Scientists have calculated this too. It turned out that such erupted “geological” water would also be just enough to fill all the oceans.
In the central parts of our planet, forming its core, there is probably no water. It is unlikely that it could exist there. Some scientists believe that further, even if oxygen and hydrogen are present there, then they must, together with other elements, form new to science, unknown metal-like forms of compounds that have a high density and are stable at the enormous pressures and temperatures that reign in the center of the globe .
Other researchers are confident that the core of the globe consists of iron. What actually is not so far from us, under our feet, at depths exceeding 3 thousand km, no one yet knows, but there is probably no water there.
Most of the water in the Earth's interior is found in its mantle - layers located under the earth's crust and extending to a depth of approximately 3 thousand km. Geologists believe that at least 13 billion cubic meters are concentrated in the mantle. km of water.
The topmost layer of the earth's shell - the earth's crust - contains approximately 1.5 billion cubic meters. km of water. Almost all the water in these layers is in a bound state - it is part of rocks and minerals, forming hydrates. You cannot bathe in this water and you cannot drink it.
The hydrosphere, the water shell of the globe, is formed by approximately another 1.5 billion cubic meters. km of water. Almost all of this amount is contained in the World Ocean. It occupies about 70% of the entire earth's surface, its area is over 360 million square meters. km. From space, our planet does not look like a globe at all, but rather like a water balloon.
The average depth of the Ocean is about 4 km. If we compare this “bottomless depth” with the size of the globe itself, the average diameter of which is equal to km, then, on the contrary, we will have to admit that we live on a wet planet, it is only slightly moistened with water, and even then not over the entire surface. The water in the oceans and seas is salty - you cannot drink it.
There is very little water on land: only about 90 million cubic meters. km. Of these, more than 60 million cubic meters. km is underground, almost all of it is salt water. About 25 million cubic meters. km of solid water lies in mountainous and glacial regions, in the Arctic, Greenland, and Antarctica. These water reserves on the globe are protected.
All lakes, swamps, man-made reservoirs and soil contain another 500 thousand cubic meters. km of water.
Water is also present in the atmosphere. There is always a lot of water vapor in the air, even in the most arid deserts, where there is not a drop of water and it never rains. In addition, clouds are always floating across the sky, clouds are gathering, it is snowing, it is raining, and fog is spreading over the ground. All these reserves of water in the atmosphere have been accurately calculated: all of them taken together amount to only 14 thousand cubic meters. km.
Positive charges in a water molecule are associated with atoms
hydrogen. Negative charges are valence electrons
oxygen. Their relative position in a water molecule can be
depicted as a simple tetrahedron.
How is an ice molecule built?
There are no special ice molecules. The molecules of water, due to their remarkable structure, are connected to each other in a piece of ice so that each of them is connected and surrounded by four other molecules. This leads to the appearance of a very loose ice structure, in which a lot of free volume remains. The correct crystalline structure of ice is expressed in the amazing grace of snowflakes and the beauty of frosty patterns on frozen window panes.

B n uzu - schematic arrangement of the atomic nuclei of hydrogen and oxygen in the water molecules that formed the crystal lattice of ice. Up- water molecules that formed an ice crystal while maintaining the scale of the electron shells. Pay attention to the loose structure of the ice.
How are water molecules built in water?
Unfortunately, this very important issue has not been studied sufficiently. The structure of molecules in liquid water is very complex. When ice melts, its mesh
the structure is partially preserved in the resulting water. The molecules in melt water consist of many simple molecules - aggregates that retain the properties of ice. As the temperature rises, some of them disintegrate and their sizes become smaller.
Mutual attraction leads to the fact that the average size of a complex water molecule in liquid water significantly exceeds the size of a single water molecule. This extraordinary molecular structure of water determines its extraordinary physicochemical properties,
At what temperature should water boil?
This question is, of course, strange. After all, water boils at one hundred degrees. Everyone knows this. Moreover, everyone knows that it is the boiling point of water at a pressure of one atmosphere that was chosen as the reference point of the temperature scale, conventionally designated 100°C.
However, the question is posed differently: at what temperature should water boil? After all, the boiling temperatures of various substances are not random. They depend on the position of the elements that make up their molecules in Mendeleev’s periodic table.
The lower the atomic number of an element, the lower its atomic weight, the lower the boiling point of its compounds. Based on its chemical composition, water can be called an oxygen hydride. H 2 Te, H 2 Se and H 2 S are chemical analogues of water. If you monitor their boiling points and compare how the boiling points of hydrides change in other groups of the periodic table, then you can quite accurately determine the boiling point of any hydride, as well as any other compound. Mendeleev himself predicted the properties of chemical compounds of elements not yet discovered in this way.
If we determine the boiling point of oxygen hydride by its position in the periodic table, it turns out that water should boil at 80° below zero. Therefore, water boils approximately one hundred and eighty degrees higher than it should boil. The boiling point of water - this is its most common property - turns out to be extraordinary and surprising.
Now try to imagine that our water has suddenly lost the ability to form complex, associated molecules. Then it would probably have to boil at the temperature it should be in accordance with the periodic law. What would happen on our Earth then? The oceans will suddenly boil. There will not be a single drop of water left on Earth, and not a single cloud will ever appear in the sky again... After all, in the atmosphere of the globe, the temperature nowhere drops below minus 80° - minus 90°C.
At what temperature does water freeze?
Isn't it true that the question is no less strange than the previous one? Well, who doesn’t know that water freezes at zero degrees? This is the second reference point of the thermometer. This is the most common property of water. But even in this case, one can ask at what temperature water should freeze in accordance with its chemical nature. It turns out that oxygen hydride, based on its position in the periodic table, would have to solidify at one hundred degrees below zero.
It is in a state of aggregation, which tends to have a gaseous or liquid form at room temperature. The properties of ice began to be studied hundreds of years ago. About two hundred years ago, scientists discovered that water is not a simple compound, but a complex chemical element consisting of oxygen and hydrogen. After discovery, the formula of water became H2O.
Ice structure
H 2 O consists of two hydrogen atoms and one oxygen atom. In a quiet state, hydrogen is located on the tops of the oxygen atom. Oxygen and hydrogen ions should occupy the vertices of an isosceles triangle: oxygen is located at the vertex of a right angle. This structure of water is called a dipole.
Ice consists of 11.2% hydrogen, and the rest is oxygen. The properties of ice depend on its chemical structure. Sometimes it contains gaseous or mechanical formations - impurities.
Ice occurs in nature in the form of a few crystalline species that stably retain their structure at temperatures from zero and below, but at zero and above it begins to melt.
Crystal structure
The properties of ice, snow and steam are completely different and depend on In the solid state, H 2 O is surrounded by four molecules located at the corners of the tetrahedron. Since the coordination number is low, the ice may have an openwork structure. This is reflected in the properties of ice and its density.

Ice shapes
Ice is one of the most common substances in nature. On Earth there are the following varieties:
- river;
- lake;
- nautical;
- firn;
- glacier;
- ground.
There is ice that is directly formed by sublimation, i.e. from the vapor state. This appearance takes on a skeletal shape (we call them snowflakes) and aggregates of dendritic and skeletal growth (frost, hoarfrost).
One of the most common forms are stalactites, i.e. icicles. They grow all over the world: on the surface of the Earth, in caves. This type of ice is formed by the flow of water droplets when the temperature difference is about zero degrees in the autumn-spring period.
Formations in the form of ice strips that appear along the edges of reservoirs, at the boundary of water and air, as well as along the edge of puddles, are called ice banks.
Ice can form in porous soils in the form of fibrous veins.
Properties of ice
A substance can be in different states. Based on this, the question arises: what property of ice is manifested in this or that state?
Scientists distinguish physical and mechanical properties. Each of them has its own characteristics.

Physical properties
The physical properties of ice include:
- Density. In physics, an inhomogeneous medium is represented by the limit of the ratio of the mass of the substance of the medium itself to the volume in which it is contained. The density of water, like other substances, is a function of temperature and pressure. Typically, calculations use a constant density of water equal to 1000 kg/m3. A more accurate density indicator is taken into account only when it is necessary to carry out very accurate calculations due to the importance of the resulting density difference result.
When calculating the density of ice, it is taken into account what kind of water has become ice: as is known, the density of salt water is higher than distilled water. - Water temperature. Usually occurs at a temperature of zero degrees. Freezing processes occur intermittently with the release of heat. The reverse process (melting) occurs when the same amount of heat is absorbed that was released, but without jumps, but gradually.
In nature, there are conditions under which water is supercooled, but it does not freeze. Some rivers retain liquid water even at a temperature of -2 degrees. - the amount of heat that is absorbed when a body is heated by each degree. There is a specific heat capacity, which is characterized by the amount of heat required to heat a kilogram of distilled water by one degree.
- Compressibility. Another physical property of snow and ice is compressibility, which affects the decrease in volume under the influence of increased external pressure. The reciprocal quantity is called elasticity.
- Ice strength.
- Ice color. This property depends on the absorption of light and the scattering of rays, as well as the amount of impurities in the frozen water. River and lake ice without foreign impurities is visible in soft blue light. Sea ice can be completely different: blue, green, blue, white, brown, or have a steely tint. Sometimes you can see black ice. It acquires this color due to a large number of minerals and various organic impurities.

Mechanical properties of ice
The mechanical properties of ice and water are determined by their resistance to the influence of the external environment relative to a unit area. Mechanical properties depend on structure, salinity, temperature and porosity.
Ice is an elastic, viscous, plastic formation, but there are conditions under which it becomes hard and very brittle.
Sea ice and freshwater ice are different: the former is much more flexible and less durable.

When passing ships, the mechanical properties of ice must be taken into account. This is also important when using ice roads, crossings and more.
Water, snow and ice have similar properties that determine the characteristics of the substance. But at the same time, these readings are influenced by many other factors: ambient temperature, impurities in the solid, as well as the initial composition of the liquid. Ice is one of the most interesting substances on Earth.